Proteomics of Ca2+-mediated signaling - PNAS Cover Feature
Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain
Aug 01, 2010
Müller CS, Haupt A, Bildl W, Schindler J, Knaus HG, Meissner M, Rammner B, Striessnig J, Flockerzi V, Fakler B, Schulte U
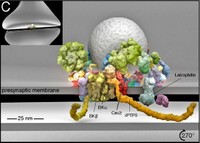
Local Ca2+ signaling occurring within nanometers of voltage-gated Ca2+ (Cav) channels is crucial for CNS function, yet the molecular composition of Cav channel nano-environments is largely unresolved. Here, we used a proteomic strategy combining knockout-controlled multiepitope affinity purifications with high-resolution quantitative MS for comprehensive analysis of the molecular nano-environments of the Cav2 channel family in the whole rodent brain.
The analysis shows that Cav2 channels, composed of pore-forming α1 and auxiliary β subunits, are embedded into protein networks that may be assembled from a pool of ∼200 proteins with distinct abundance, stability of assembly, and preference for the three Cav2 subtypes.
The majority of these proteins have not previously been linked to Cav channels; about two-thirds are dedicated to the control of intracellular Ca2+ concentration, including G protein-coupled receptor-mediated signaling, to activity-dependent cytoskeleton remodeling or Ca2+-dependent effector systems that comprise a high portion of the priming and release machinery of synaptic vesicles.
The identified protein networks reflect the cellular processes that can be initiated by Cav2 channel activity and define the molecular framework for organization and operation of local Ca2+ signaling by Cav2 channels in the brain.